| Author |
Message |
yahoo2

Guru

Joined: 05/04/2011
Location: AustraliaPosts: 1166 |
| Posted: 04:41pm 03 Sep 2012 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
Promised to do this a long time ago, finally making a start. The wind outside is 56 Kmh gusting to 85Kmh so I'd rather be in here tapping the keyboard.
I have a small stockpile of clear sided batteries with various problems from age and abuse. I will post some photos and make a few comments about what I think has caused them.
This first one is a battery originally designed for backup power. It is kept fully charged for months on end waiting for that one day when it is needed. The first thing you notice is the massive amount of fluid that it holds in relation to the area of the plates. reason???it's able to use a lower acid concentration to reduce oxidisation (essentially rust) but still have enough acid to carry out the reaction. made for low power delivery over extended periods of time. Checking the specific gravity shows a charged level of 1.225 - 1.230. This is close to what I would expect for this use. There is a specific gravity meter (broken)and thermometer built into the case, handy for quickly looking for a fault

The positive plate on these will be a lead grid that is packed with PbO2 (lead dioxide and lots of other goodies) that is held in place with plastic spacers and porous fiberglass wraps. This is designed to let the acid flow through and complete the reaction, leave room for the bulkier and less densely packed lead sulphate to form and to stop the lead dioxide from shedding away from the positive plate and falling out of the grid into the bottom of the battery. You can only see the negative parallel electrode connectors in this photo, both it and the positive one are in good nick, they are massively built, not like a car battery that is just a few MM thick.

Looking at the positive plate here I can see a number of things. The tops of the grids are splintering and the plastic compression/separator plates have split, none of the dark black material is electrically connected to the plate anymore so it is basically sitting in acid with bubbles of oxygen flowing up past it, it has lost its dark grey lead look and gone black because it is badly rusted.

The white areas on the fibreglass woven fabric are where it has been stretched and torn from a massive buildup of Pbso4 expanding throughout the positive grids. At some stage these batteries have been kept in an undercharged or uncharged state for a long period of time and the harder crystalline structure has put a lot of pressure on the layers that hold the plate together.

Considering their age, these batteries are surprisingly clean in the bottom, I have seen them with muck right up to the bottom of the plates, shorting them out. The light grey colour is a layer of lead silt from degradation at the negative plate, I don't understand how, over time atoms of lead are detached from the plate, I doubt it is a water contamination issue (calcium, etc) just old age. The bits that look like burnt matchsticks are from the top of the positive plates.Edited by yahoo2 2012-09-05
I'm confused, no wait... maybe I'm not... |
| |
yahoo2

Guru

Joined: 05/04/2011
Location: AustraliaPosts: 1166 |
| Posted: 06:08pm 03 Sep 2012 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
These two photos were tough to get. What we see here is lead sulphate growing as a crystal strand. Unusual to see it so clearly on a solid lead plate and to this length, way better than I hoped for. This is considered to be the good form of PbSO4 structure, very easy to recombine and react.


Notice how big the positive plate is here, it is supposed to be below the level of the white separator (same level as the negative in front of it) it has been expanded more than 4 centimeters and is almost pushed into the large bar joining all the negative plates. In this battery the yellow on the negative electrode is a sulphur byproduct and the dark brown colour just to the right of it on the positive plate is not "red lead", it is a mild oxidation (bit hard to see, I will get a better shot later).Edited by yahoo2 2012-09-05
I'm confused, no wait... maybe I'm not... |
| |
yahoo2

Guru

Joined: 05/04/2011
Location: AustraliaPosts: 1166 |
| Posted: 01:29am 04 Sep 2012 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|

I'm confused, no wait... maybe I'm not... |
| |
Wombat

Regular Member

Joined: 27/05/2011
Location: AustraliaPosts: 72 |
| Posted: 08:09am 04 Sep 2012 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
Wow... What an amazing post!
I am seriously lost for words!
Superb pics with your see through batterys...
If only all batterys were made this way we could
all see what's going on inside our own systems.
I really hope you get some good usage from them.
Thanks for the post and watch with interest.
Russ.
 |
| |
mac46

Guru

Joined: 07/02/2008
Location: United StatesPosts: 412 |
| Posted: 01:16pm 04 Sep 2012 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
Do you think that some normal service use and regular recharges will help or hurt the battery condition?
Mac46
I'm just a farmer |
| |
Georgen
Guru

Joined: 13/09/2011
Location: AustraliaPosts: 462 |
| Posted: 06:23pm 04 Sep 2012 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
Suppose transparent materials were not as robust as dark ones, but I too would love to have see-through plastic on my battery.
I could quickly react to something happening inside, not to mention that checking water level would take 10 seconds, compared to opening every cell to have a look at water level.
George |
| |
yahoo2

Guru

Joined: 05/04/2011
Location: AustraliaPosts: 1166 |
| Posted: 08:56pm 04 Sep 2012 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
The batteries I am photographing are anything from 30 to 50 years old, I've just kept them for interests sake. I wish I had taken some photos when I first got them because there was some really weird looking stuff going on.
This one is interesting, it is some recent rusting probably due to low water levels exposing the tops of the plates. If you look closely, there is hard crystals growing in the surface of the freshly exposed metal.

Negative plate sponge lead, nuf said!
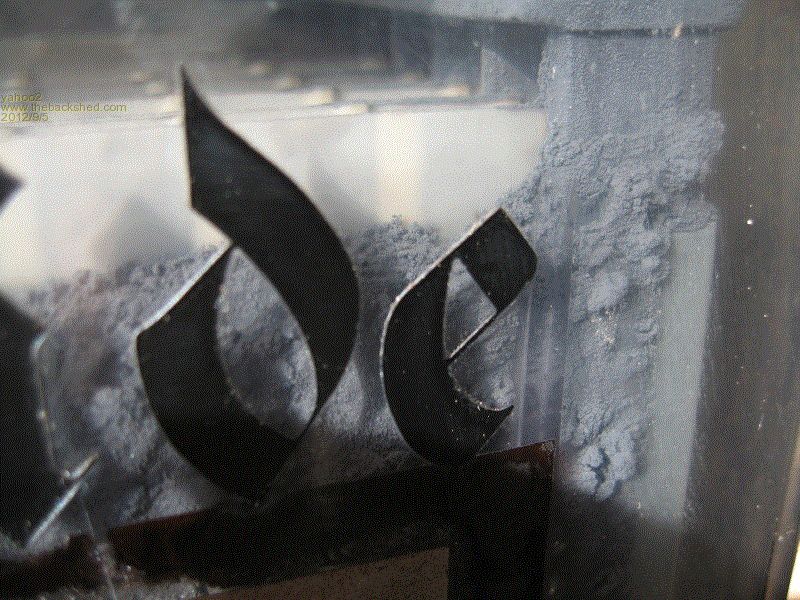
I'm confused, no wait... maybe I'm not... |
| |
yahoo2

Guru

Joined: 05/04/2011
Location: AustraliaPosts: 1166 |
| Posted: 09:30pm 04 Sep 2012 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
Right, this one is unusual, it is from two banks of six in series with a couple of cells that were badly gassing from low resistance across some plates. This cell was alongside one of the faulty ones and it looks like it has discharged in reverse.

That is actually the negative electrode and plates that is growing lead sulphate.

Three forms of sulphate here, the patch on the left looks like a furry mould, there are tendrils of what looks like fluffy strings of cotton hanging from the plates bottom edge and blobs and pimples stuck to the face of the plate.

Wide shot

Thin layer of PbSO4 formed due to stratification of the acid probably grown on the top of a layer of water or very dilute acid. The bright white layer is very dense and the area underneath is starting to crowd with material like the roots of a plant in a pot.

I'm confused, no wait... maybe I'm not... |
| |
yahoo2

Guru

Joined: 05/04/2011
Location: AustraliaPosts: 1166 |
| Posted: 09:47pm 04 Sep 2012 |
 Copy link to clipboard Copy link to clipboard |
 Print this post |
|
When I look at a battery bank for the first time, this is one of the things I look for.
Lifting around the positive terminal. Normally have to look very closely and compare it with the negative terminal to see a difference.

I'm confused, no wait... maybe I'm not... |
| |

